The Role of Trypsin in Digestion and Biotechnology: An In-Depth Exploration
When it comes to understanding the intricacies of digestion and its applications in biotechnology, one enzyme takes center stage – trypsin. This remarkable enzyme plays a pivotal role in breaking down proteins, facilitating digestion in our bodies, and revolutionizing various biotechnological processes. In this comprehensive exploration, we’ll delve into the multifaceted world of trypsin, its mechanisms, applications, and significance in both biology and industry.
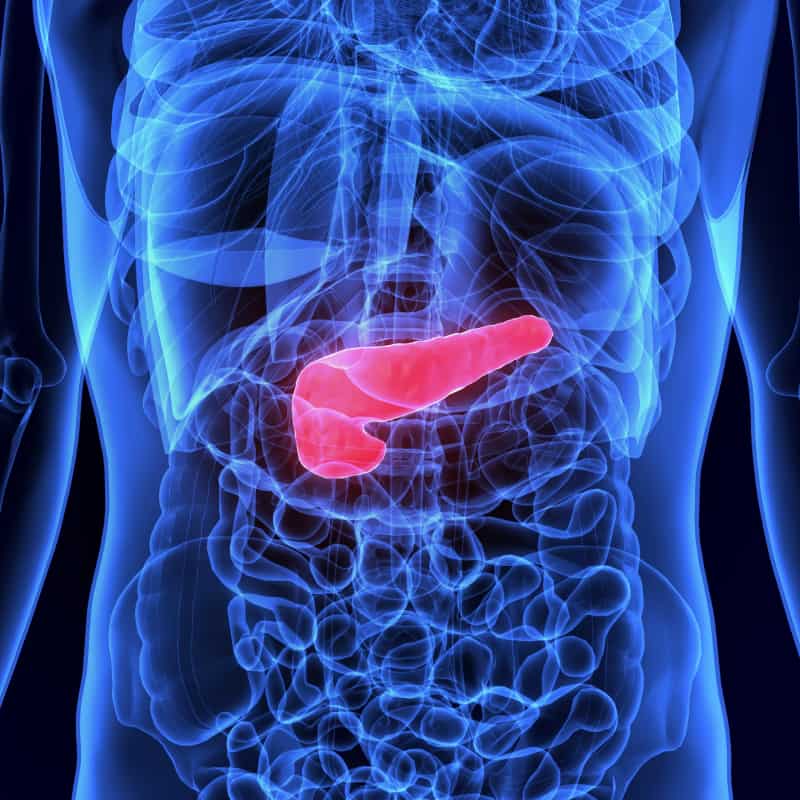
Overview of Trypsin
What is Trypsin?
Trypsin is a key enzyme found in the digestive system of humans and many other organisms. It is part of a group of enzymes known as proteases, which specialize in breaking down proteins into smaller components, called peptides. Trypsin’s unique ability to target specific peptide bonds within proteins makes it a crucial player in digestion.
How Does Trypsin Work in the Digestive System?
The digestive journey of trypsin begins in the pancreas, where it is produced as an inactive precursor called trypsinogen. Trypsinogen is then released into the small intestine, where it meets another enzyme called enterokinase. Enterokinase triggers the conversion of trypsinogen into its active form, trypsin.
Once activated, trypsin sets to work. It cleaves peptide bonds, primarily targeting those on the carboxyl side of the amino acids lysine and arginine. This cleavage results in the formation of smaller peptides, which are further broken down by other enzymes like chymotrypsin and carboxypeptidase, ultimately yielding amino acids that can be absorbed and utilized by the body.
Trypsin in Digestion
The Significance of Trypsin Activation
The activation of trypsin is a tightly regulated process that prevents premature enzyme activity within the pancreas. The presence of trypsinogen and enterokinase in separate cellular compartments ensures that trypsin activation occurs only after trypsinogen has been safely transported to the small intestine.
Trypsinogen to Trypsin Conversion: A Protective Mechanism
The conversion of trypsinogen to trypsin is a well-orchestrated process to prevent autodigestion of the pancreas. If trypsin were to become active prematurely within the pancreas, it could lead to pancreatic damage. This protective mechanism ensures that trypsin is activated only at the site where it is needed for digestion.
Trypsin in Biotechnology
Beyond its crucial role in digestion, trypsin has found a multitude of applications in the realm of biotechnology. Its ability to cleave specific peptide bonds makes it an indispensable tool in various processes.
Cell Culture and Passaging
One of the primary applications of trypsin in biotechnology is in cell culture. Researchers use trypsin to detach adherent cells from culture surfaces, allowing for the passage of cells from one culture vessel to another. This process is essential for cell expansion and propagation in laboratories, particularly in fields like regenerative medicine and vaccine development.
Protein Purification
Trypsin’s specificity in cleaving peptide bonds has made it a vital component in protein purification techniques. Researchers use trypsin to digest complex protein mixtures, resulting in a collection of peptide fragments. These fragments can then be analyzed and sequenced, aiding in the identification and purification of specific proteins. Techniques like mass spectrometry rely on trypsin digestion to determine the sequence and structure of proteins.
Medical Relevance of Trypsin
While trypsin plays a critical role in digestion and biotechnology, deficiencies in trypsin production or activation can have medical implications. Trypsin deficiency can lead to various health issues, including impaired digestion and malabsorption of nutrients.
Trypsin Production and Sources
Natural Sources
Trypsin is naturally produced in various organisms, including humans. It is primarily synthesized in the pancreas, where it is stored as trypsinogen until needed for digestion. Once released into the small intestine, trypsinogen is converted to its active form, trypsin, as part of the digestive process.
Commercial Production
In addition to natural sources, trypsin is produced commercially for research and medical purposes. Commercial trypsin is typically derived from animal sources, such as bovine or porcine pancreas. It undergoes rigorous purification processes to ensure its quality and safety for use in laboratories and medical settings.
Frequently Asked Questions About Trypsin
Q: What is trypsin, and what role does it play in digestion?
A: Trypsin is an enzyme that plays a crucial role in digestion by breaking down proteins into smaller peptides, aiding in their absorption in the small intestine.
Q: How is trypsin activated in the digestive system?
A: Trypsin is activated in the small intestine when trypsinogen, its inactive precursor, encounters the enzyme enterokinase. Enterokinase triggers the conversion of trypsinogen into active trypsin.
Q: What makes trypsin different from other digestive enzymes?
A: Trypsin’s unique specificity allows it to cleave peptide bonds at specific amino acids (lysine and arginine), making it distinct from other proteases involved in digestion.
Q: How is trypsin used in cell culture?
A: Trypsin is used in cell culture to detach adherent cells from culture surfaces, facilitating cell passaging and propagation in laboratory settings.
Q: Can trypsin be used for protein purification?
A: Yes, trypsin is commonly used for protein purification. It digests complex protein mixtures, producing peptide fragments that can be analyzed and sequenced for protein identification.
Q: Are there medical conditions related to trypsin deficiency?
A: Yes, trypsin deficiency can lead to medical issues, including impaired digestion, chronic diarrhea, weight loss, and malnutrition due to poor nutrient absorption.
Q: Is elevated trypsin a biomarker for specific medical conditions?
A: Elevated trypsin levels can be indicative of pancreatic disorders such as pancreatitis or pancreatic cancer and are used as biomarkers for diagnosis and monitoring.
Q: What are the natural sources of trypsin in the body?
A: Trypsin is naturally produced in the pancreas and is released into the small intestine for digestion when needed.
Q: How is commercial trypsin produced, and what are its sources?
A: Commercial trypsin is derived from animal sources, primarily bovine or porcine pancreas, and undergoes purification processes to ensure quality and safety.
Q: What safety precautions should be taken when working with trypsin in laboratories?
A: Safety measures include wearing appropriate personal protective equipment, avoiding contact with skin and eyes, and following lab protocols for handling and disposal.
Conclusion:
Trypsin, often overshadowed by its better-known digestive counterparts, is an unsung hero in both our bodies and the world of biotechnology. Its precise role in breaking down proteins during digestion ensures efficient nutrient absorption, while its applications in cell culture and protein purification drive advancements in biotechnology and medical research. Understanding trypsin’s significance in both biology and industry sheds light on the intricate web of processes that sustain life and propel scientific discovery. As we continue to unravel its mysteries, trypsin remains a remarkable enzyme that bridges the gap between our fundamental biological needs and cutting-edge technology.